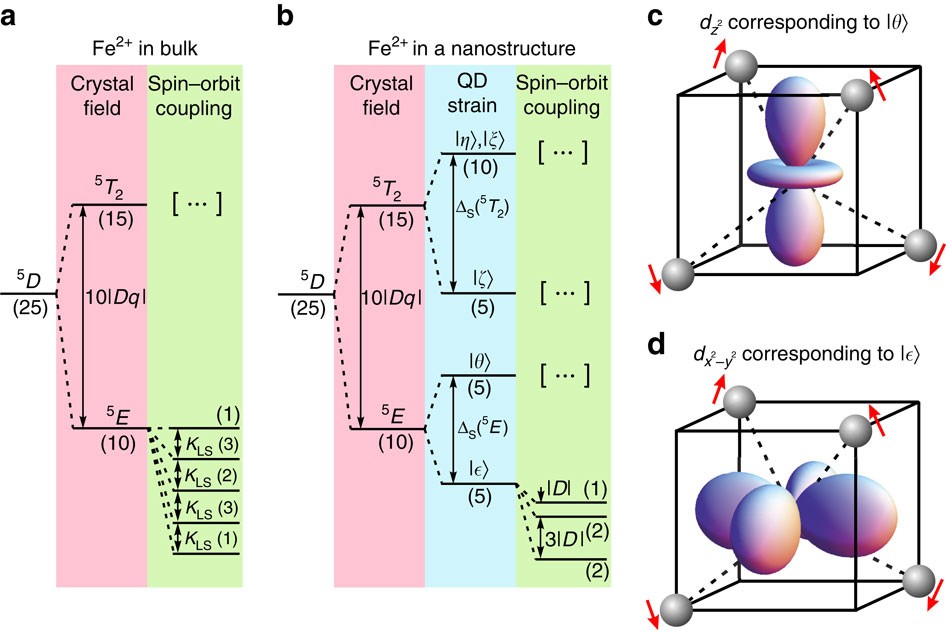
Magnetic ground state of an individual Fe2+ ion in strained semiconductor nanostructure | Nature Communications
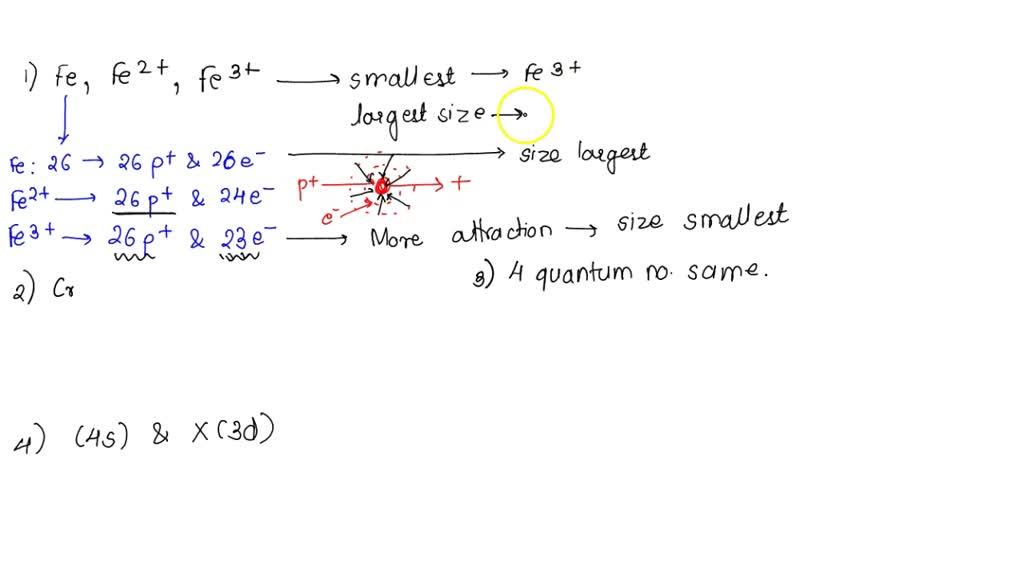
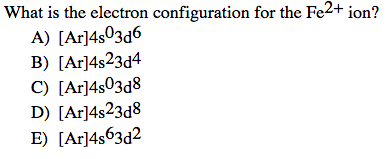
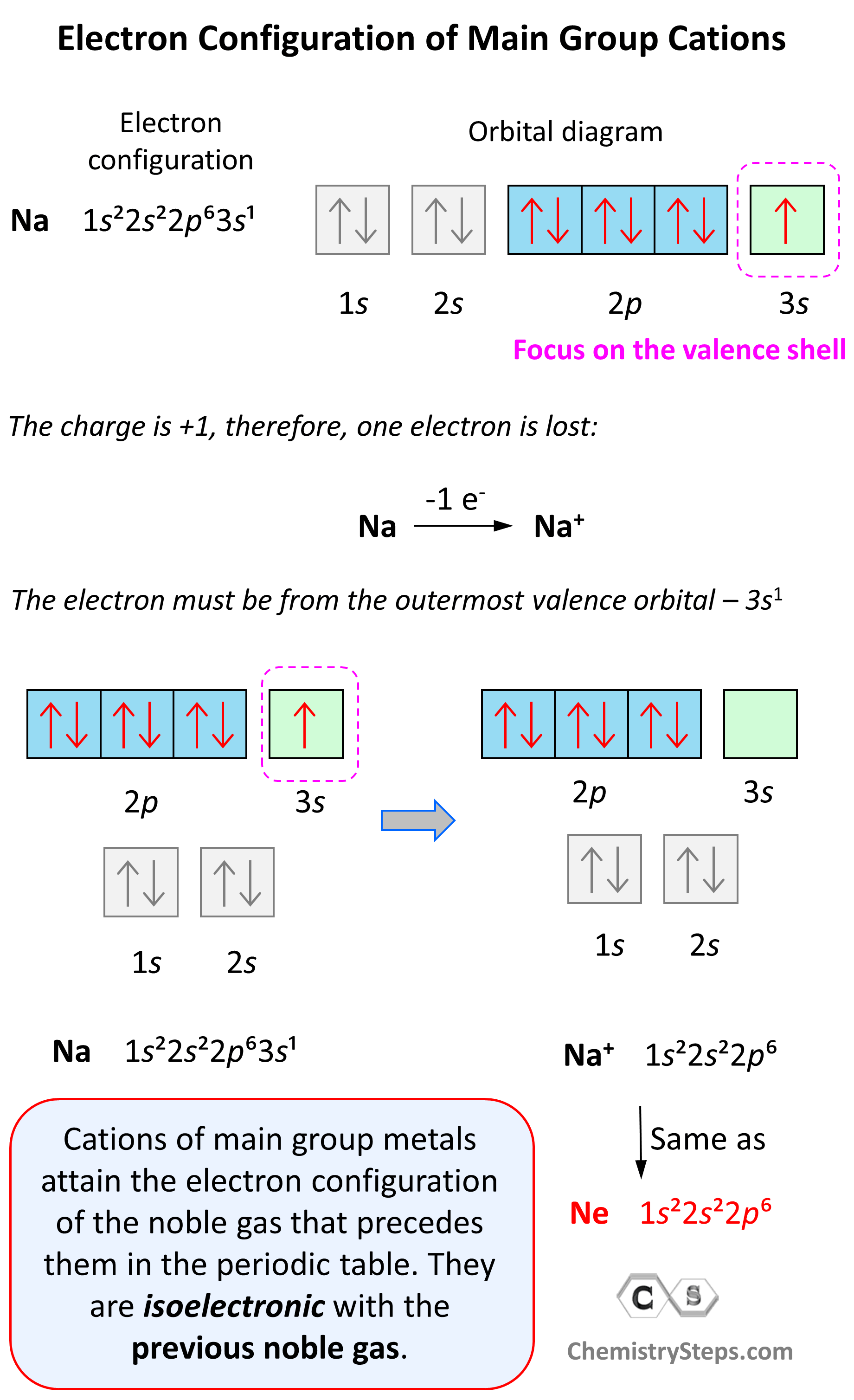
SOLVED: 1. With respect to size, rank Fe, Fe2+, Fe3+ from smallest to largest and explain. 2. What is the reason for Chromium's unexpected electron configuration. 3. Why can no 2 electron

Ionic radius and electron configuration for (a) Fe; (b) Fe +2 and (c)... | Download Scientific Diagram

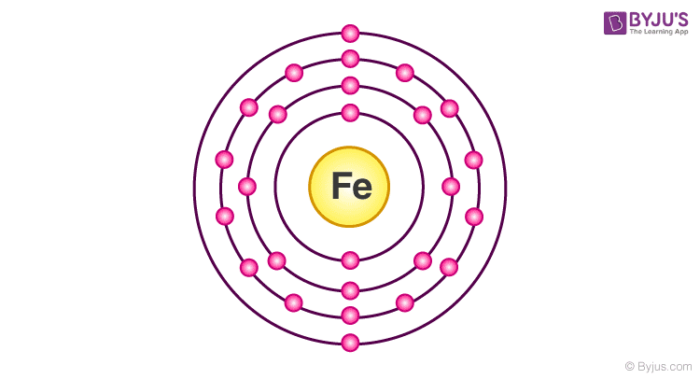
I my chemistry book electron configuration of of iron is 2 8 14 2 but by using formula of 2 - Brainly.in

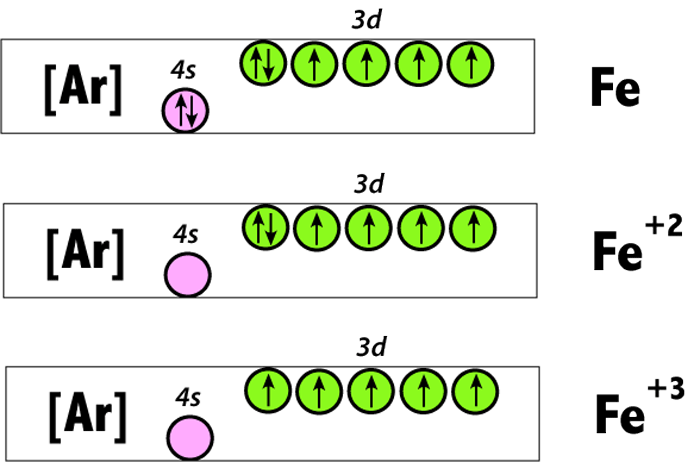
Fe^2+ ,Fe^3+ and Fe Electron Configuration (Two Ways)-Crash Course Chemistry | Electron configuration, Crash course, Electrons
![Draw orbital box diagrams for Fe^2+, Fe^3+, Zn, and Zn^2+. Tell which is paramagnetic. [Paramagnetic means that it has unpaired electrons. This can only be seen with box diagrams.] | Homework.Study.com Draw orbital box diagrams for Fe^2+, Fe^3+, Zn, and Zn^2+. Tell which is paramagnetic. [Paramagnetic means that it has unpaired electrons. This can only be seen with box diagrams.] | Homework.Study.com](https://homework.study.com/cimages/multimages/16/3-3409192489036411813.jpg)
Draw orbital box diagrams for Fe^2+, Fe^3+, Zn, and Zn^2+. Tell which is paramagnetic. [Paramagnetic means that it has unpaired electrons. This can only be seen with box diagrams.] | Homework.Study.com