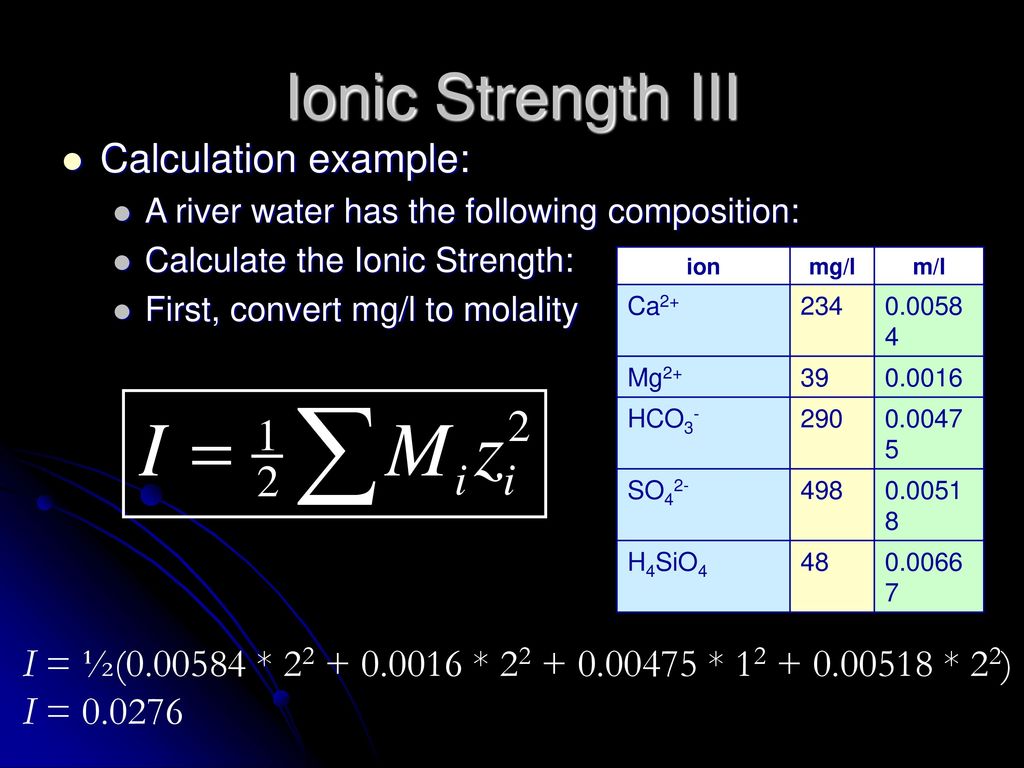
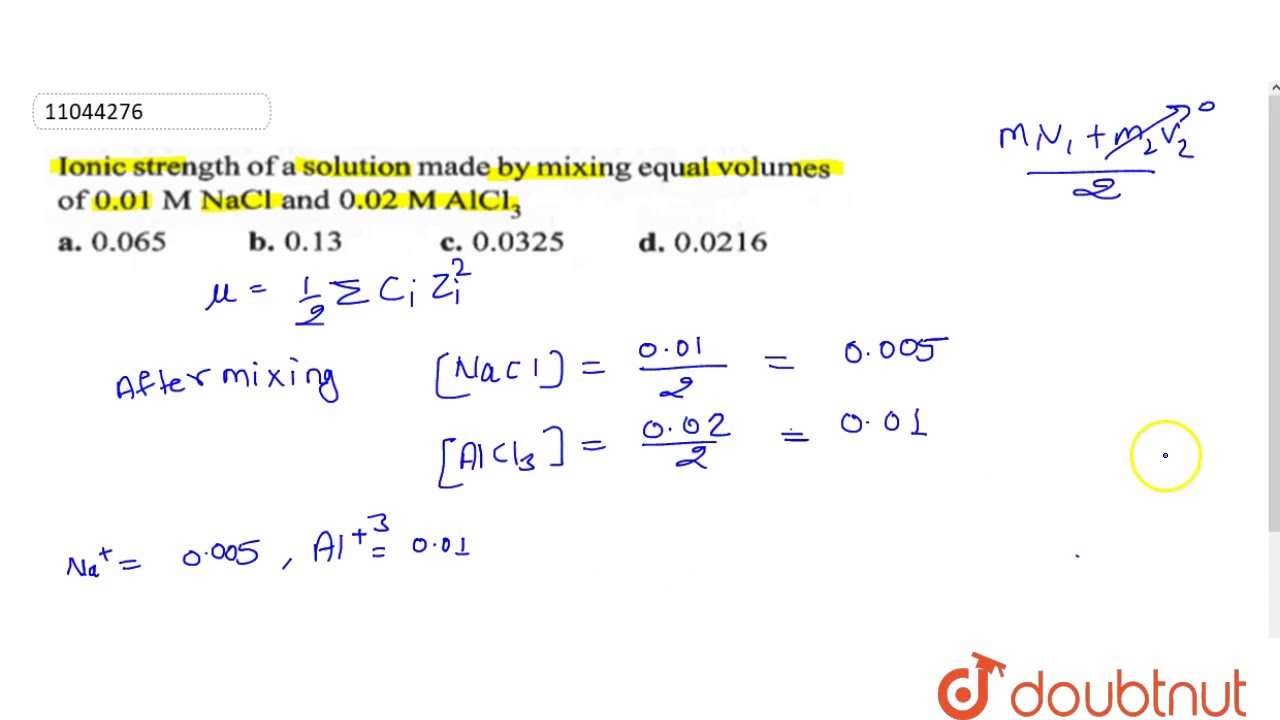
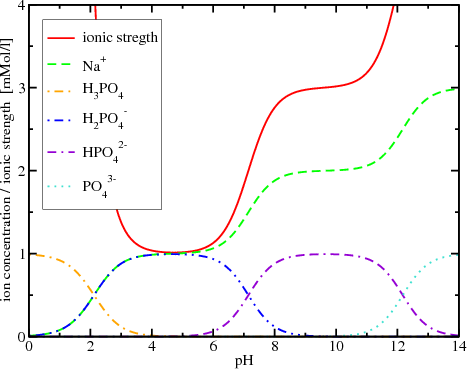
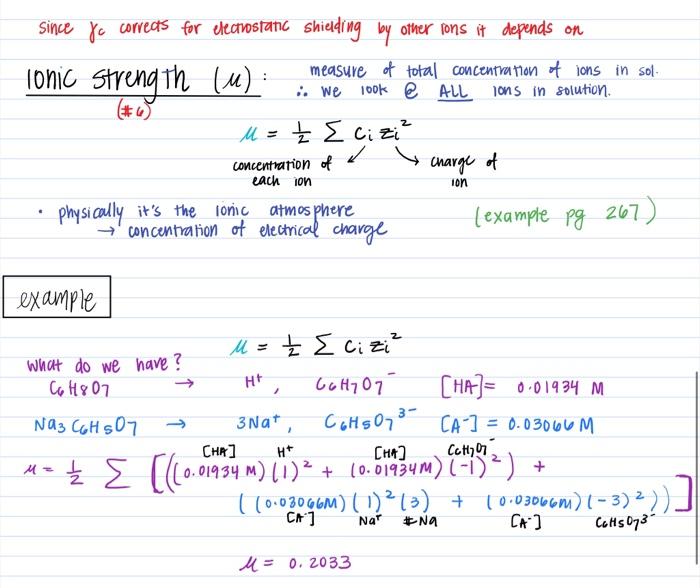
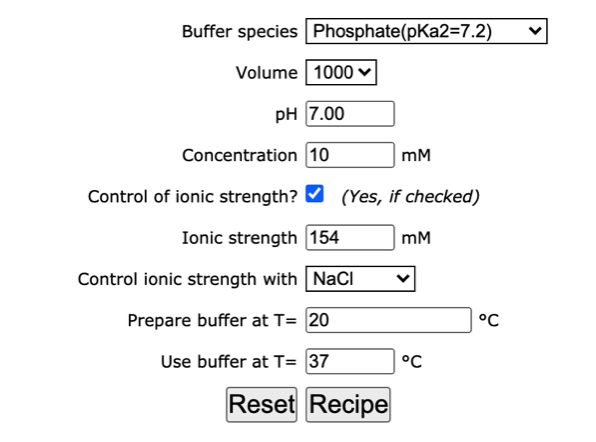
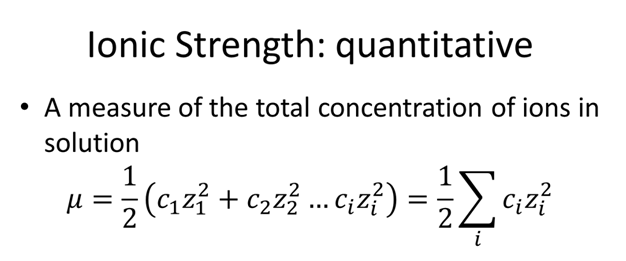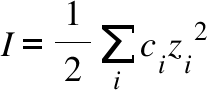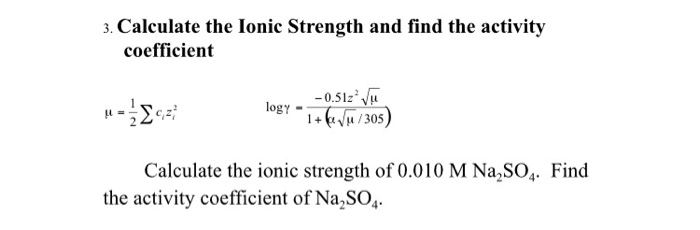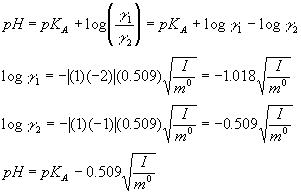Ionic strength - Solved problems-electrochemistry-calculation-example-IIT JEE NEET JAM CSIR NET GATE - YouTube
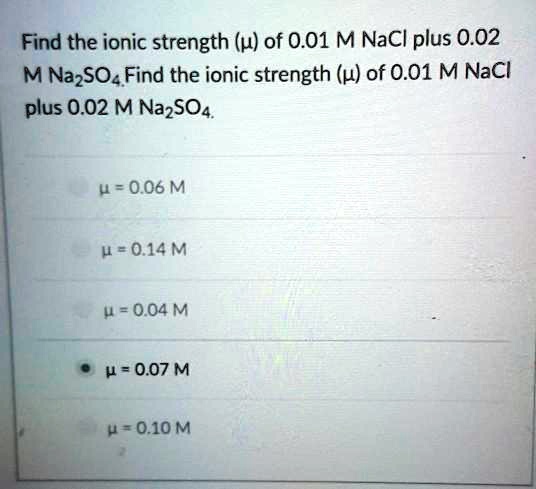
SOLVED: Find the ionic strength (u) of 0.01 M NaCl plus 0.02 M Na2SO4 0 = 0.06 M 0.20 M 4 = 0.04 M 4 = 0.07 M = 0.10 M

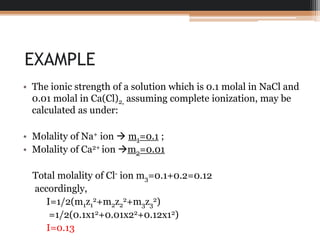
Question No. 4 The ionic strength of a solution containing 0.1 mole/kg of KCl and 0.2 mole/ kg of Cuso, is (A) 0.3 (B) 0.6 (C) 0.9 (D) 0.2
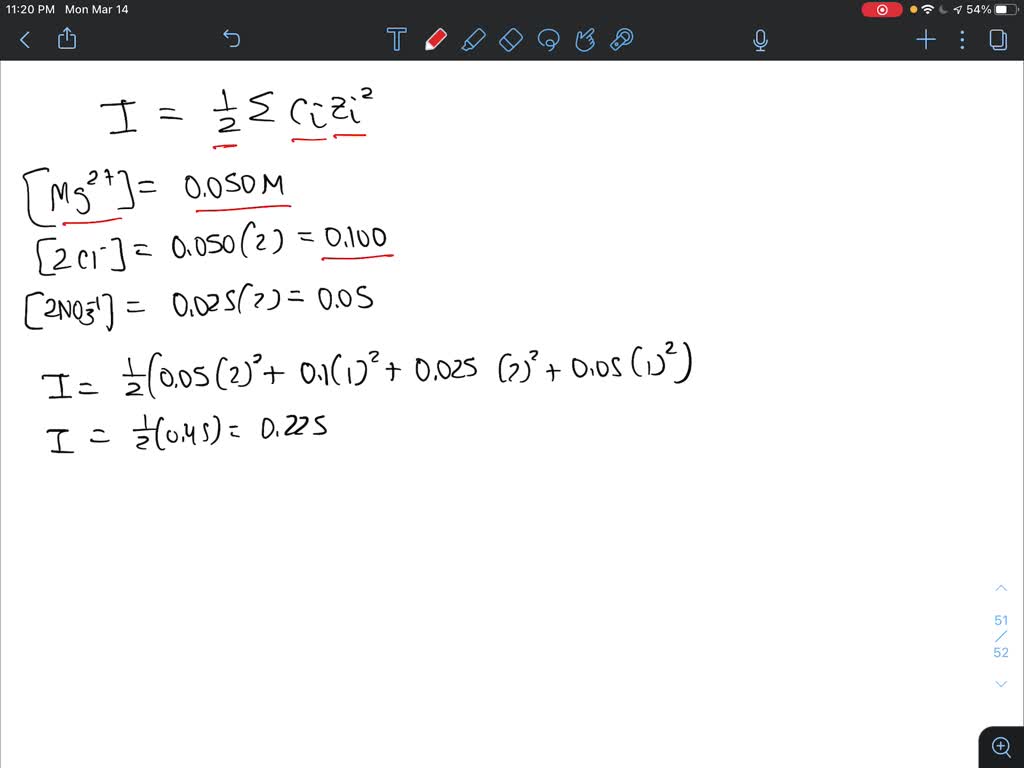
SOLVED: Calculate the ionic strength of a solution that contains 0.050 M magnesium chloride and 0.025 M magnesium nitrate

pH calculations and more in fundamentals of pharmaceutics. : What is ionic strength of solutions and how is it calculated?

Ionic strength - Solved problems-electrochemistry-calculation-example-IIT JEE NEET JAM CSIR NET GATE

Find the ionic strength of (Electrochemistry): i. 0.05 \ mol \ dm^{-3} \ KCl(aq) ii. 0.05 \ mol \ kg^{-1} LaFe(CN)_6(ag) Give detail explanation. (eq. why does CN have a charge of

Find the ionic strength of (Electrochemistry): i. 0.05 \ mol \ dm^{-3} \ KCl(aq) ii. 0.05 \ mol \ kg^{-1} LaFe(CN)_6(ag) Give detail explanation. (eq. why does CN have a charge of