Present Periodic Table showing elements up to the critical Z cr which... | Download Scientific Diagram

Confused by notation of atomic number Z and mass number A on periodic table of elements - Chemistry Stack Exchange

Suggested Periodic Table Up To Z ≤ 172, Based on Dirac–Fock Calculations, 2011. | Periodic table, Internet database, Save

Pyykkö's periodic table extended to Z = 172 (with permission from PCCP... | Download Scientific Diagram
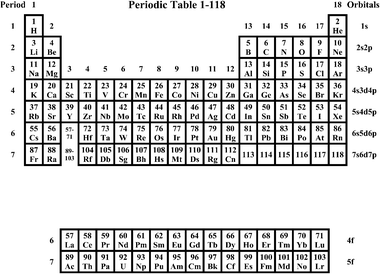
A suggested periodic table up to Z ≤ 172, based on Dirac–Fock calculations on atoms and ions - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C0CP01575J

BeWise Classes Modern Periodic Table of Elements Wall Chart Educational Poster for Students | Thick Laminated, Waterproof & Dust Resistant Non ...

Atomic number, Z, increases across (left-to-right) and down the Janet... | Download Scientific Diagram
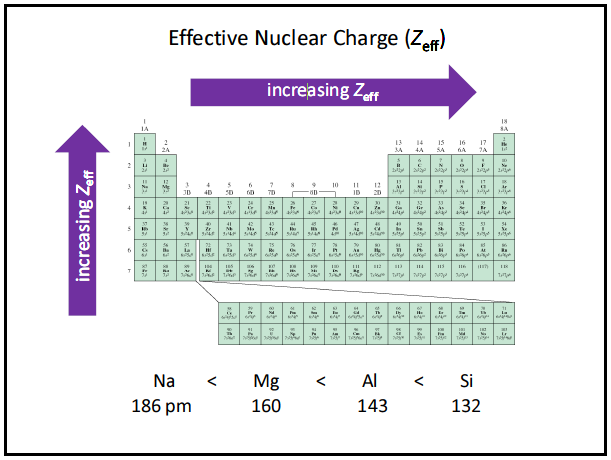


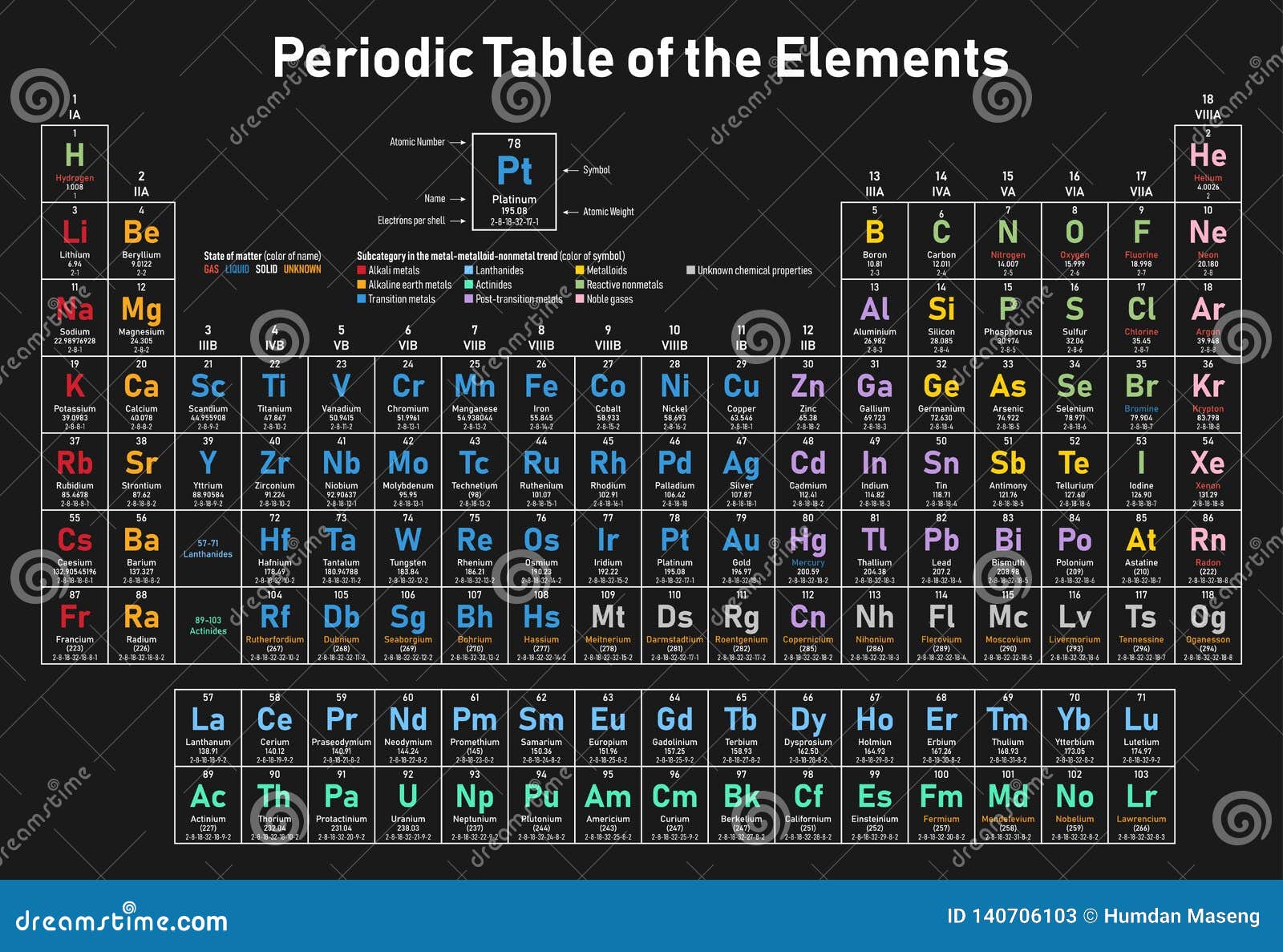



![Solution] Periodic Trends: Effective Nuclear Charge | Wizeprep Solution] Periodic Trends: Effective Nuclear Charge | Wizeprep](https://d3rw207pwvlq3a.cloudfront.net/attachments/000/059/758/original/image.png?1566758136)